Concrete, in its simplest form, is a mixture of paste and aggregates. The paste, composed of portland cement and water, coats the surface of the fine and coarse aggregates. Through a chemical reaction, the paste hardens and gains strength to form the rock-like mass known as concrete.
Concrete is durable, strong and relatively low cost , which makes it the backbone of buildings and infrastructure world-wide. Houses, schools and hospitals as well as airports, bridges, highways and rail systems and more all use concrete, making it most-produced material on Earth. Concrete will only be more in demand as developing nations become increasingly urban, extreme weather events necessitate more durable building materials and the price of other infrastructure materials continues to rise.
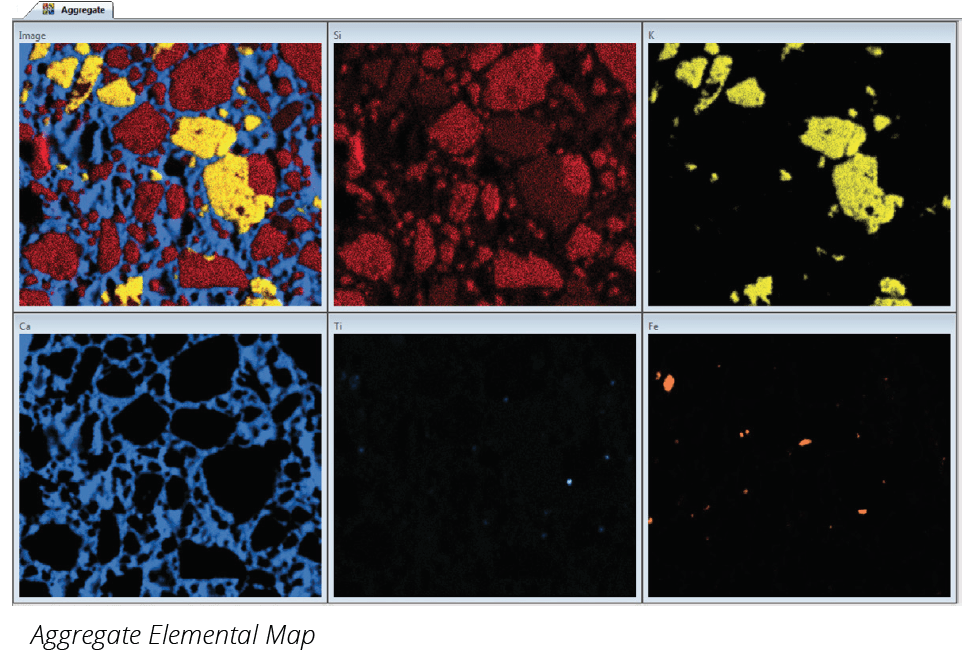
It is common to incorrectly use the terms cement and concrete interchangeably. Cement is an ingredient used to make the concrete. Cement is the fine powder that, when mixed with water, sand, and gravel or crushed stone (fine and coarse aggregate), forms concrete. Portland cement, the most common type of cement, is made by mining, and then grinding raw materials that include limestone, clay, and bauxite to a fine powder, called raw meal. This is then heated in a rotating cement kiln. This process produces clinker: rounded nodules between 1mm and 25mm across. Routine chemical analysis is an essential part in controlling the manufacturing of cement, from the full analysis of raw materials to testing each stage of the process. Specifically, the composition of clinker has to be closely monitored to ensure the quality of the cement.
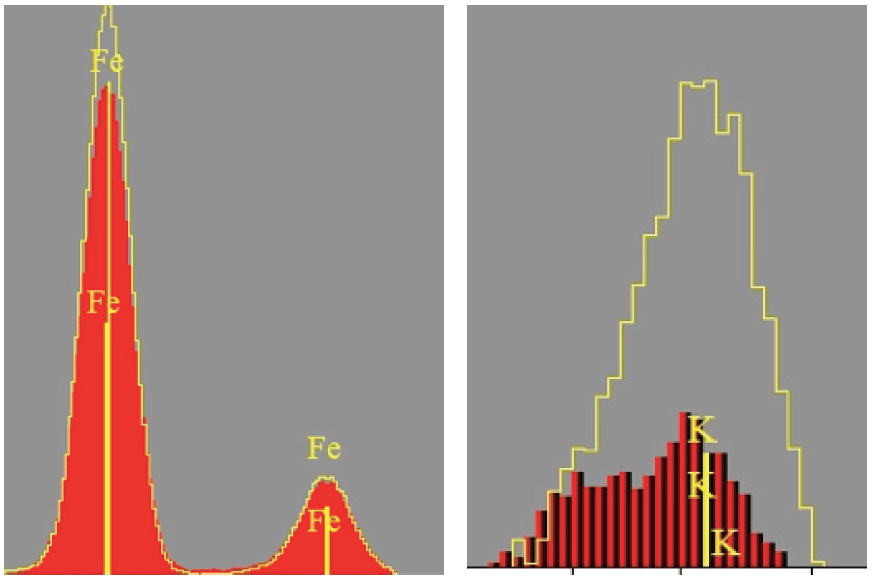
Excess free lime results in undesirable effects such as volume expansion, increased setting time or reduced strength. Free lime must be monitored constantly during the process to allow the operator to determine and maintain the optimum operating temperature of the kiln in order to obtain maximum reactivity and to reduce thermal consumption. Control of the basic reaction requires accurate analysis for at least calcium, silicon, aluminum, and iron in the raw meal.
Fast and accurate cement analysis is required for today’s cement manufacturing industry.
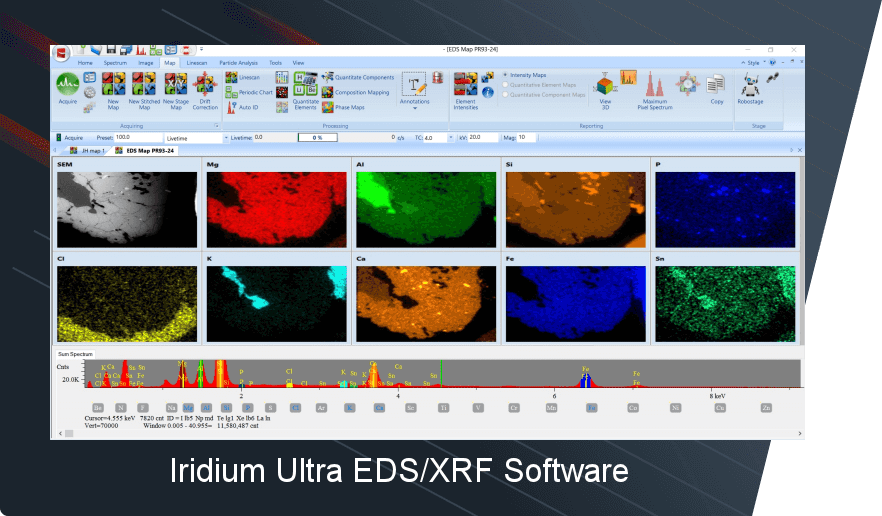
X-Ray fluorescence (XRF) spectrometry is one of the simplest instrumental techniques for analysis in a cement works because there is no sample preparation and results can be reviewed minutes after a sample is taken. XRF analysis can be used at many stages of the cement analysis process, from raw materials, i.e. at the quarry, to intermediate products (clinker, gypsum, limestone) and the finished product (cement). But also, waste materials are increasingly being used in the cement making process. When they contain the main cement making components, waste materials can replace some of the natural raw materials and combustible wastes. This means more testing of the incoming raw materials, both during the process and in the final product.
ATLAS provides quick and accurate chemical composition analysis, with no sample preparation, allowing the cement to be continuously produced on a 24/7 basis with no delays or reworks.