History
The electron microprobe, also known as the electron probe microanalyzer, developed utilizing two technologies: electron microscopy — the use of a focused high energy electron beam to interact with a target material, and X-ray spectroscopy — identification of the photons resulting from electron beam interaction with the target, with the energy/wavelength of the photons being characteristic of the atoms excited by the incident electrons. The names of Ernst Ruska and Max Knoll are associated with the first prototype electron microscope in 1931. The name of Henry Moseley is associated with the discovery of the direct relationship between the wavelength of X-rays and the identity of the atom from which it originated[6].
There have been at several historical threads to electron beam microanalytical technique. One was developed by James Hillier and Richard Baker at RCA. In the early 1940s, they built an electron microprobe, combining an electron microscope and an energy loss spectrometer.[7] A patent application was filed in 1944. Electron energy loss spectroscopy is very good for light element analysis and they obtained spectra of C-Kα, N-Kα and O-Kα radiation. In 1947, Hiller patented the idea of using an electron beam to produce analytical X-rays, but never constructed a working model. His design proposed using Bragg diffraction from a flat crystal to select specific X-ray wavelengths and a photographic plate as a detector. However, RCA had no interest in pursuing commercialization of this invention.
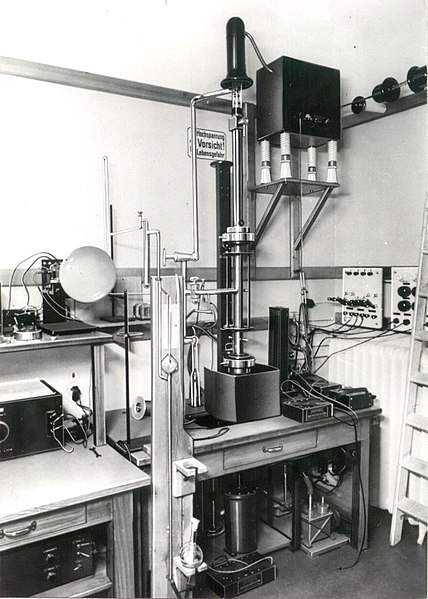
A second thread developed in France in the late 1940s. In 1948–1950, Raimond Castaing, supervised by André Guinier, built the first electron “microsonde électronique” (electron microprobe) at ONERA. This microprobe produced an electron beam diameter of 1-3 μm with a beam current of ~10 nanoamperes (nA) and used a Geiger counter to detect the X-rays produced from the sample. However, the Geiger counter could not distinguish X-rays produced from specific elements and in 1950, Castaing added a quartz crystal between the sample and the detector to permit wavelength discrimination. He also added an optical microscope to view the point of beam impact. The resulting microprobe was described in Castaing’s 1951 PhD Thesis,[8], translated into English by Pol Duwez and David Wittry [9], in which he laid the foundations of the theory and application of quantitative analysis by electron microprobe, establishing the theoretical framework for the matrix corrections of absorption and fluorescence effects. Castaing (1921-1999) is considered the “father” of electron microprobe analysis.
The 1950s was a decade of great interest in electron beam X-ray microanalysis, following Castaing’s presentations at the First European Microscopy Conference in Delft in 1949[10] and then at the National Bureau of Standards conference on Electron Physics[11] in Washington, DC, in 1951, as well as at other conferences in the early to mid-1950s. Many researchers, mainly material scientists, began to develop their own experimental electron microprobes, sometimes starting from scratch, but many times utilizing surplus electron microscopes.
One of the organizers of the Delft 1949 Electron Microscopy conference was Vernon Ellis Cosslett at the Cavendish Laboratory at Cambridge University, a center of research on electron microscopy[12], as well as scanning electron microscopy with Charles Oatley as well as X-ray microscopy with Bill Nixon. Peter Duncumb combined all three technologies and developed a scanning electron X-ray microanalyzer as his PhD thesis project (published 1957), which was commercialized as the Cambridge MicroScan instrument.
Pol Duwez, a Belgian material scientist who fled the Nazis and settled at the California Institute of Technology and collaborated with Jesse DuMond, encountered André Guinier on a train in Europe in 1952, where he learned of Castaing’s new instrument and the suggestion that CalTech build a similar instrument. David Wittry was hired to build such an instrument as his PhD thesis, which he completed in 1957. It became the prototype for the ARL[13] EMX electron microprobe.
During the late 1950s and early 1960s there were over a dozen other laboratories in North America, the United Kingdom, Europe, Japan and the USSR developing electron beam X-ray microanalyzers.
The first commercial electron microprobe, the “MS85” was produced by CAMECA (France) in 1956. It was soon followed in the early-mid 1960s by many microprobes from other companies. In addition, many researchers build electron microprobes in their labs. Significant subsequent improvements and modifications to microprobes included scanning the electron beam to make X-ray maps (1960), the addition of solid state Si(Li) LN2-cooled detectors (1968) and the development of synthetic multilayer diffracting crystals for analysis of light elements (1984).
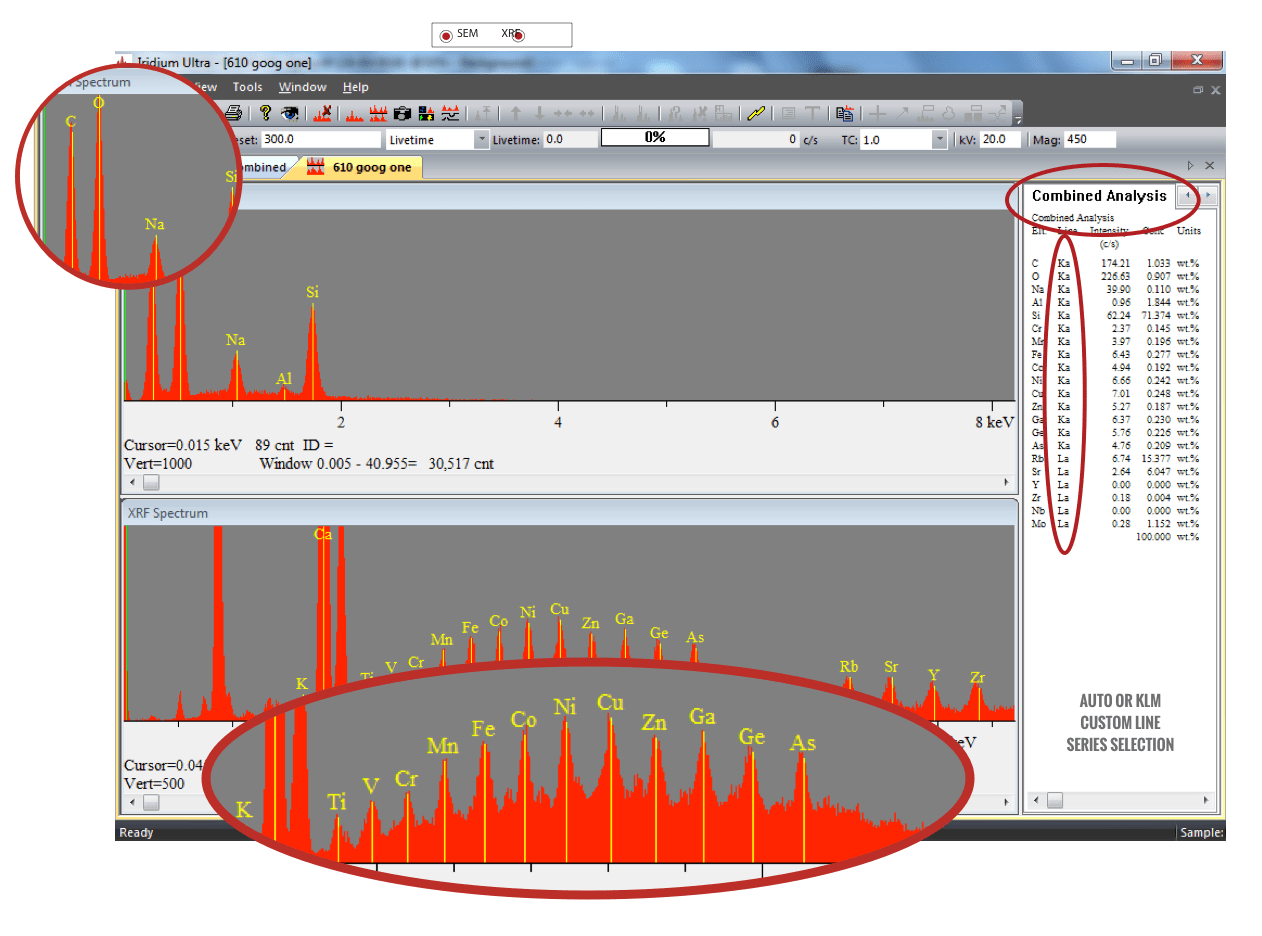
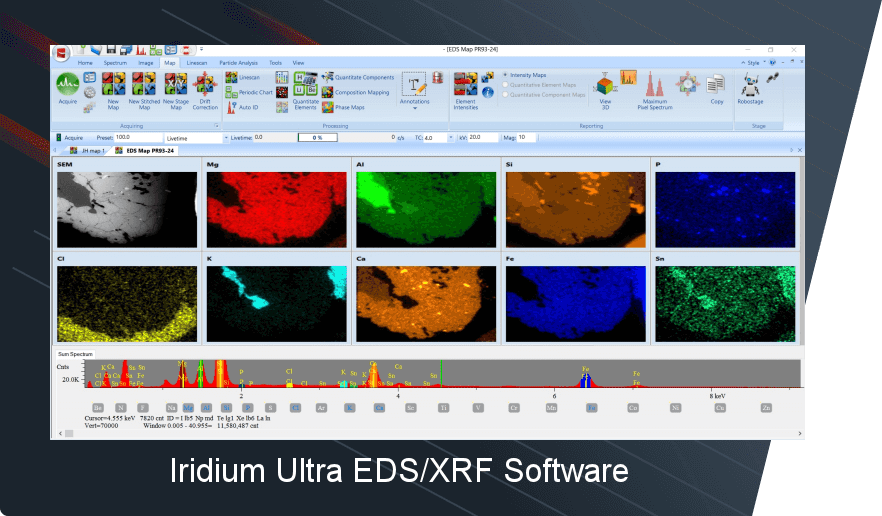
Since the late-1990’s, a newer EDS detector – called the silicon drift detector (SDD) – has superseded the Si(Li) detector systems. The SDD consists of a high-resistivity silicon chip where electrons are driven to a small collecting anode. The advantage lies in the extremely low capacitance of this anode, thereby utilizing shorter processing times and allowing very high throughput. Benefits of the SDD include:
- High count rates and processing,
- Better resolution than traditional Si(Li) detectors at high count rates,
- Lower dead time (time spent on processing X-ray event),
- Faster analytical capabilities and more precise X-ray maps or particle data collected in seconds,
- Ability to be stored and operated at relatively high temperatures, eliminating the need for liquid nitrogen cooling.
Because the capacitance of the SDD chip is independent of the active area of the detector, much larger SDD chips can be utilized (50 mm2 or more). This allows for even higher count rate collection. Further benefits of large area chips include:
- Minimizing SEM beam current allowing for optimization of imaging under analytical conditions,
- Reduced sample damage and
- Smaller beam interaction and improved spatial resolution for high speed maps.
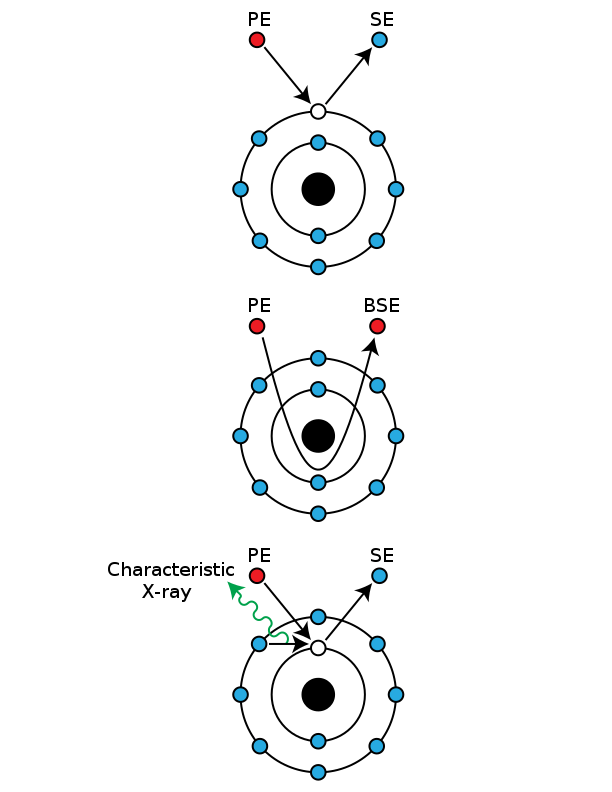
 SEM Schematic
SEM Schematic Interaction Volume
Interaction Volume